Quality leaders in the clinical laboratory space know better than anyone how essential audits are to the health of their organizations (and the stakeholders and constituents they serve). Not only do effective audits help ensure regulatory compliance, but they also provide visibility into the everyday details of your laboratories—and give you a way to troubleshoot issues before they snowball into compliance nightmares that could affect your laboratory accreditation.
Stated differently, effective audits can drive organizational excellence.
What does it mean to run “effective” audits? Many companies still rely on manual audit processes, such as paper forms and spreadsheets, to collect and manage audit data. These manual methods can be time-consuming and error-prone, and they don’t always make it easy for quality professionals to spot trends, issue timely reports, and see the big picture.
Manual processes may get the job done, but they may also limit your ability to take a proactive approach to quality. With the help of pioneering technologies and a background in laboratory audits and accreditation, ARMATURE has built an innovative suite of risk management tools that can improve how you manage your internal, compliance, and customer audits and manage critical issues.
ARMATURE Fabric™ is a powerful software platform designed to help you reach your most ambitious quality goals while boosting performance and productivity across the organization.
Audit Management Software with High Standards
ARMATURE Fabric is the result of nearly two decades spent automating audit and accreditation processes. Our software supports leading standards developing organizations and organizations that audit tissue transplants, blood testing, human pathology, and reference materials (using industry standards such as ISO/IEC 17025, ISO 17034, and ISO 15189). The software we built to support laboratory accreditation reflects our intimate understanding of how standards are measured and how structured audits are conducted in the laboratory environment.
Because every audit begins with standards, our audit management software makes it easy for you to plug in your standards, and then it brings them up through different endpoints throughout the system. You can reference one or more criteria (standards and best practices) from pre-built catalogs, or add your own configurable text. Add ratings and findings to each item, weight ratings to help measure effectiveness, and allow each criterion to support document upload, commenting, and issues/non-conformances. This way, you can collect data, documentation, and evidence during your audits and tie those artifacts to the specific ISO, FDA, or other standards you’re evaluating against.
Like the rest of ARMATURE’s integrated risk management tools, our audit management software is highly configurable, so you can update your data collection instruments as standards change and evolve—with no coding required. We designed our software to be intuitive and easy-to-use because we understand that user adoption is a crucial ingredient for software success.
Plug in your standards and pull them up at different endpoints throughout our system.
Build Your Audits to Get the Information You Need
In addition to working with standards developing organizations, we have collaborated with leading conformity assessment bodies to streamline and automate their audit/assessment processes. Our experience in this area has shaped ARMATURE Fabric’s web-based audit management tools.
Quality leaders can take advantage of our intuitive, highly configurable platform to build audit checklists and assessments, with help from our team as needed. Our system allows you to choose from a number of question types including text (short form, narrative), multiple choice, number, date, and document upload. You can also set a number of options on your questions, such as required, read-only, no-data, data keys, variables, field names, and help. Our software allows you to model your existing forms and checklists, or build new ones from the ground up. Either way is user-friendly and easy to update, so you can make your audit software reflect your quality goals, compliance needs, and organizational objectives—even as they change over time.
Build an audit that auto-populates standards.
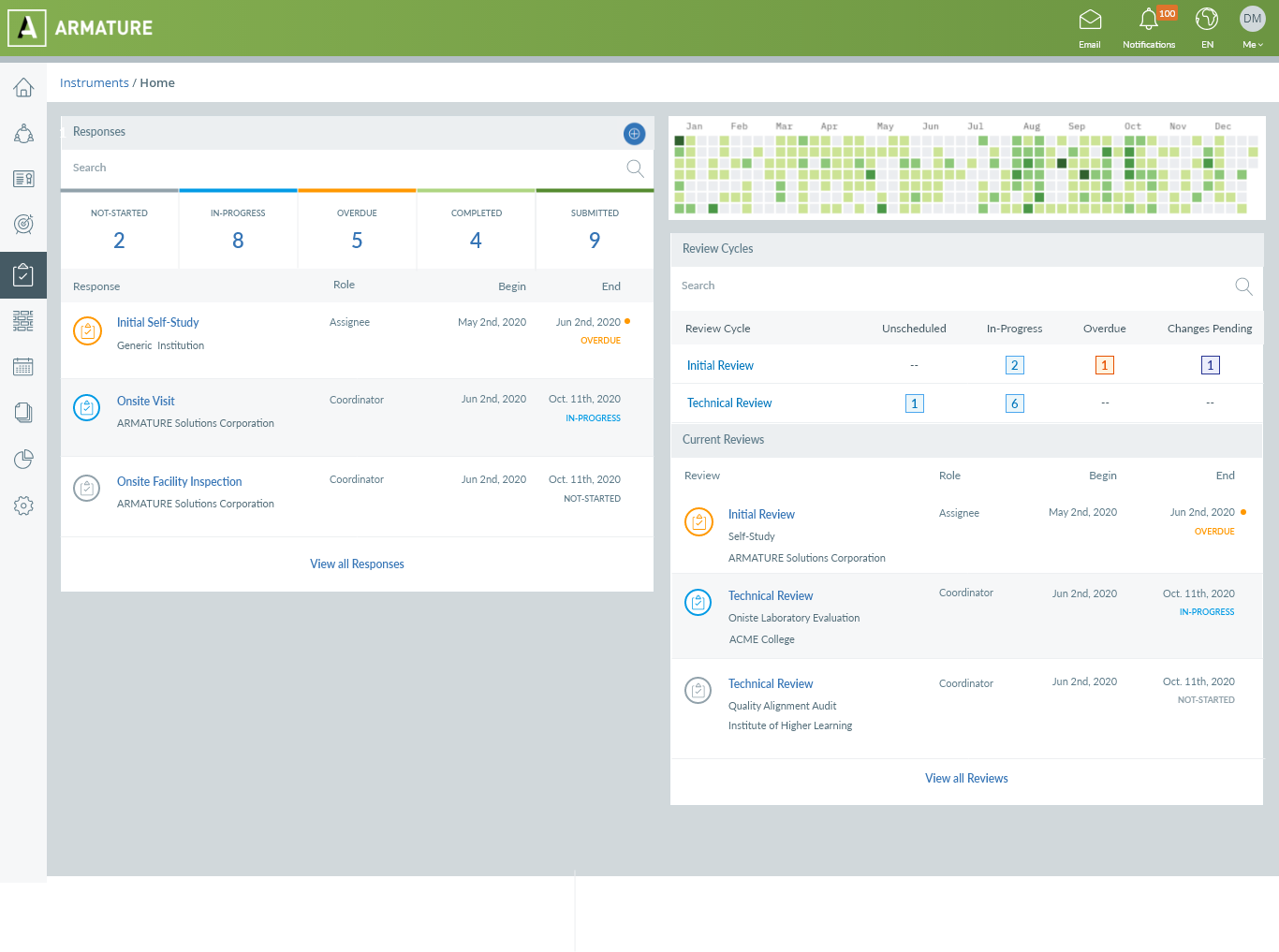
Working either online or offline, auditors can use ARMATURE’s audit management software to evaluate criteria, log findings, answer questions, review and respond to rich content, and submit their information for review. Our audit tools allow auditors to upload photos and documentation, create comments and discussions, and capture issues and non-conformances as they run through the assessment process. The software flattens these findings into data that you can shape into visualizations and reports.
It’s a way of mining audits for all of the insights they contain—so that you can take a proactive approach to quality that helps your organization ensure regulatory compliance and truly get ahead.
Visualize Your Audit Data in Real-Time
When quality data is imprisoned in spreadsheet cells, it can be a challenge to visualize the impact of your audit findings. You’re tasked with running a range of clinical laboratory audits to ensure ISO/IEC or FDA compliance, which may sometimes make you feel like you’re playing whack-a-mole with your audits: focusing on the most urgent issues that bubble up, without the luxury of taking a step back to survey the landscape for trends and improvement opportunities.
ARMATURE Fabric’s audit management software gives you the visibility and control you need through a series of configurable charts and graphs that bring your audit data to life in real-time. When an auditor logs an issue during an audit, our software feeds the findings into intuitive visualizations, so you’ll see real-time updates that you can use to get a snapshot understanding of your audit landscape, or export to built-in report templates for sharing. Freed from their spreadsheet shackles, your audit findings can paint a clear picture of quality across your organization.
Like the rest of the ARMATURE Fabric environment, our data visualizations are dynamic. You can click through any issue and take immediate action to remediate it. Our powerful visualizations give you a global view of your laboratory audits, and allow you to drill down to the granular level to better understand the details of audit findings. This ability to zoom in and out, and to take action on the spot and in the moment, empowers quality leaders to make informed decisions to keep compliance efforts on track for events such as laboratory accreditation.
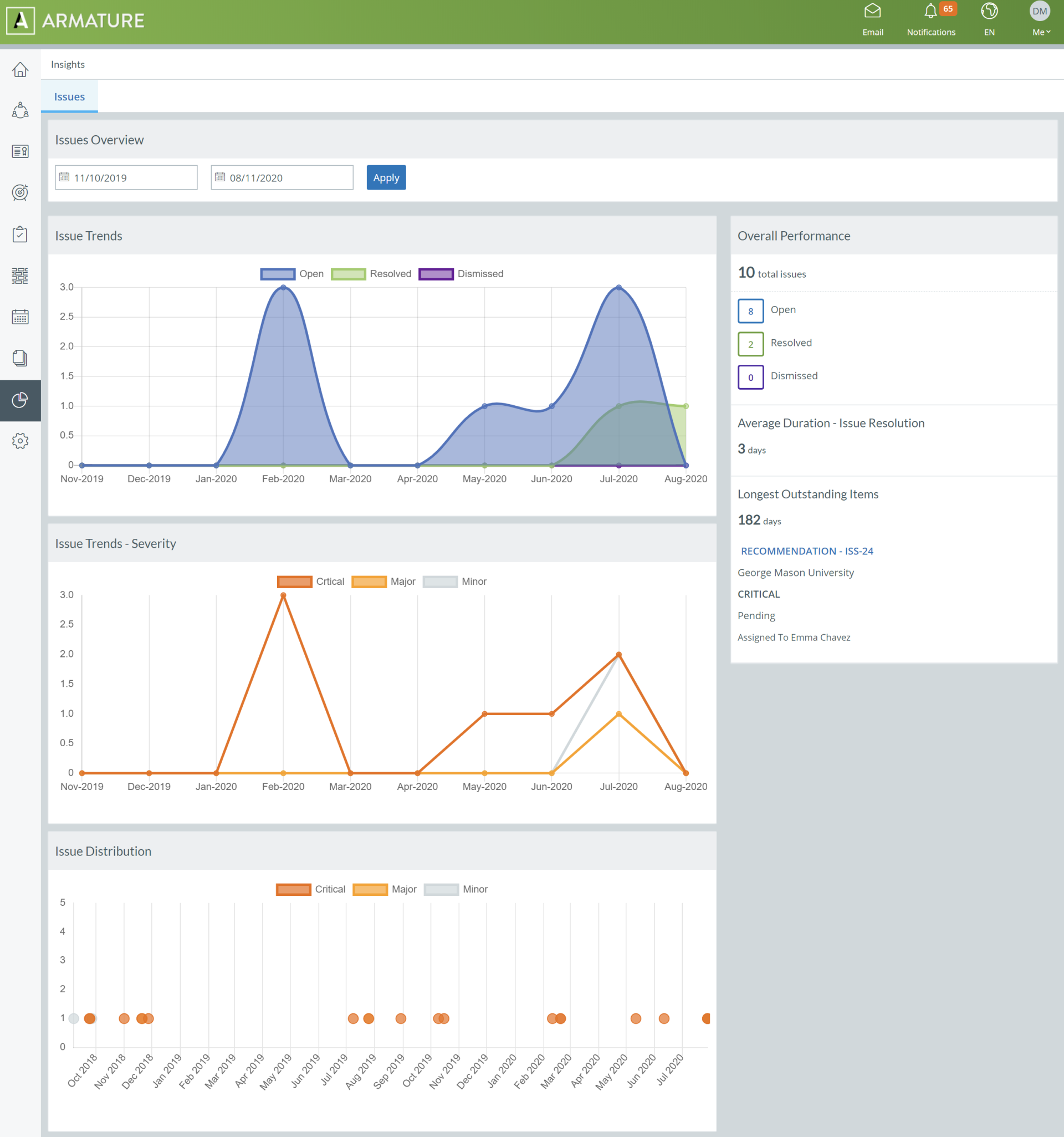
Charts & graphs update in real-time to bring your audit data to life.
Let’s Have a Quality Conversation
What auditing challenges do you face today? What’s working well in your organization, and what would you like to improve? We would love to learn more about your quality vision, and to show you how our audit management software can support your quality and compliance goals. Contact us to start a quality conversation!


